US approves first new Alzheimer's drug in 20 years
World 07:33 PM - 2021-06-07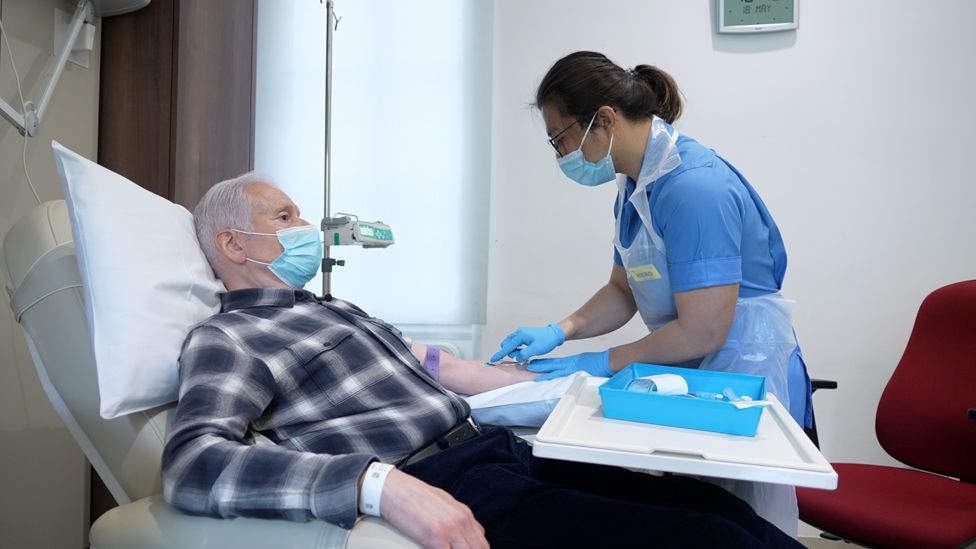
Photo Credit: BBC
The first new treatment for Alzheimer's disease for nearly 20 years has been approved by regulators in the United States, paving the way for its use in the UK.
Aducanumab targets the underlying cause of Alzheimer's, the most common form of dementia, rather than its symptoms.
Charities have welcomed the news of a new therapy for the condition.
But scientists are divided over its potential impact because of uncertainty over the trial results.
At least 100,000 people in the UK with a mild form of the disease could be suitable for the drug if it were to be approved by the UK regulator.
The US Food and Drug Administration (FDA) said there was "substantial evidence that aducanumab reduces amyloid beta plaques in the brain" and that this "is reasonably likely to predict important benefits to patients".
In March 2019, late-stage international trials of aducanumab, involving about 3,000 patients, were halted when analysis showed the drug, given as a monthly infusion, was not better at slowing the deterioration of memory and thinking problems than a dummy drug.
But later that year, the US manufacturer Biogen analysed more data and concluded the drug did work, as long as it was given in higher doses. The company also said it significantly slowed cognitive decline.
Aducanumab targets amyloid, a protein that forms abnormal clumps in the brains of people with Alzheimer's that can damage cells and trigger dementia, including:
memory and thinking problems
communication issues
confusion
PUKmedia / BBC
More news
-
German Forces Commander Ended his Mission at Mam Jalal's Grave
12:28 PM - 2024-04-23 -
Golden Bla Awards Ceremony Takes Place in Sulaymaniyah
11:32 AM - 2024-04-23 -
PUK Official: PUK is Committed to Holding Elections on Time
11:04 AM - 2024-04-23 -
Turkish President Meets Kurdish Officials in Erbil
10:42 AM - 2024-04-23
see more
U.S. State Department Mentions the Deterioration of Journalists' Rights in Iraq & Kurdistan
06:48 PM - 2024-04-25
10 Notable Individuals Receive Golden Bla Award
09:27 PM - 2024-04-23
DPM Talabani Asks Turkish President to Lift Ban on Sulaymaniyah Airport
11:43 AM - 2024-04-23
Iraqi & Turkish Presidents: Problems Should Be Resolved Through Dialogue
05:00 PM - 2024-04-22
Most read
-
U.S. State Department Mentions the Deterioration of Journalists' Rights in Iraq & Kurdistan
Reports 06:48 PM - 2024-04-25 -
Another International Report Mentions Kurdistan's Limited Freedom
Reports 09:24 PM - 2024-04-25




.jpg)
 Application
Application